HISTORY
The Power of a Shared Vision
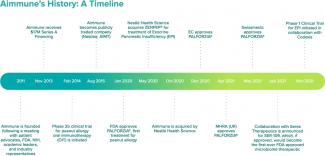
Aimmune was founded to address the need for approved treatments for food allergies, starting with peanut allergy.
Aimmune Therapeutics was founded directly in response to a united call to action by leading stakeholders in food allergy.
An advocacy-sponsored research retreat was held in 2011 that aimed to reach consensus on the direction of food allergy treatment research. There, parents of children with serious and potentially life-threatening food allergies, patient advocacy organizations, leading clinical and academic physicians, representatives from the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH), as well as members of the pharmaceutical industry, concluded there was a significant unmet need for a standard oral immunotherapy (OIT) approach and treatments.
This consensus-driven community ultimately led to the formation of the Allergen Research Corporation, the forerunner to Aimmune Therapeutics, to address this need and fulfill a unified vision.